How Bacteria Outsmart Our Best Drugs?
[Communications Biology | (2024) 7:1051]
Structural shifts in a tiny channel protein help microbes pump out life-saving antibiotics. Antibiotic resistance is often described as a looming catastrophe, but the battle isn’t fought only in hospital wards. It also plays out at the atomic scale, deep inside bacterial membranes. There, a protein called TolC acts as a molecular escape hatch, helping E. coli and other Gram-negative bacteria expel dangerous compounds, including the β-lactam antibiotics that form the backbone of modern medicine.
TolC is no ordinary protein. It is a barrel-shaped channel that spans the bacterium’s outer membrane, linking up with partner proteins inside the cell to form powerful efflux pumps. These pumps can spit out a dizzying array of antibiotics, rendering many treatments ineffective. But TolC is also a double-edged sword: while it keeps poisons out, it can also serve as an entry point for viruses and bacterial toxins. Evolution, the researchers note, has tuned TolC into a delicate balancing act.
To probe how TolC opens and closes, we turned to molecular dynamics simulations, atom-by-atom movies of the protein in action. We discovered that even in its “resting” state, TolC is primed with collective motions that naturally favor pumping molecules outward. Then, using steered molecular dynamics, we pulled three different antibiotics, oxacillin, piperacillin, and carbenicillin, through TolC’s channel, watching where the drugs stuck and where they slipped through. Oxacillin, it turned out, slid out easily. Carbenicillin, by contrast, tangled in a web of hydrogen bonds, explaining why bacteria struggle to pump it out efficiently.
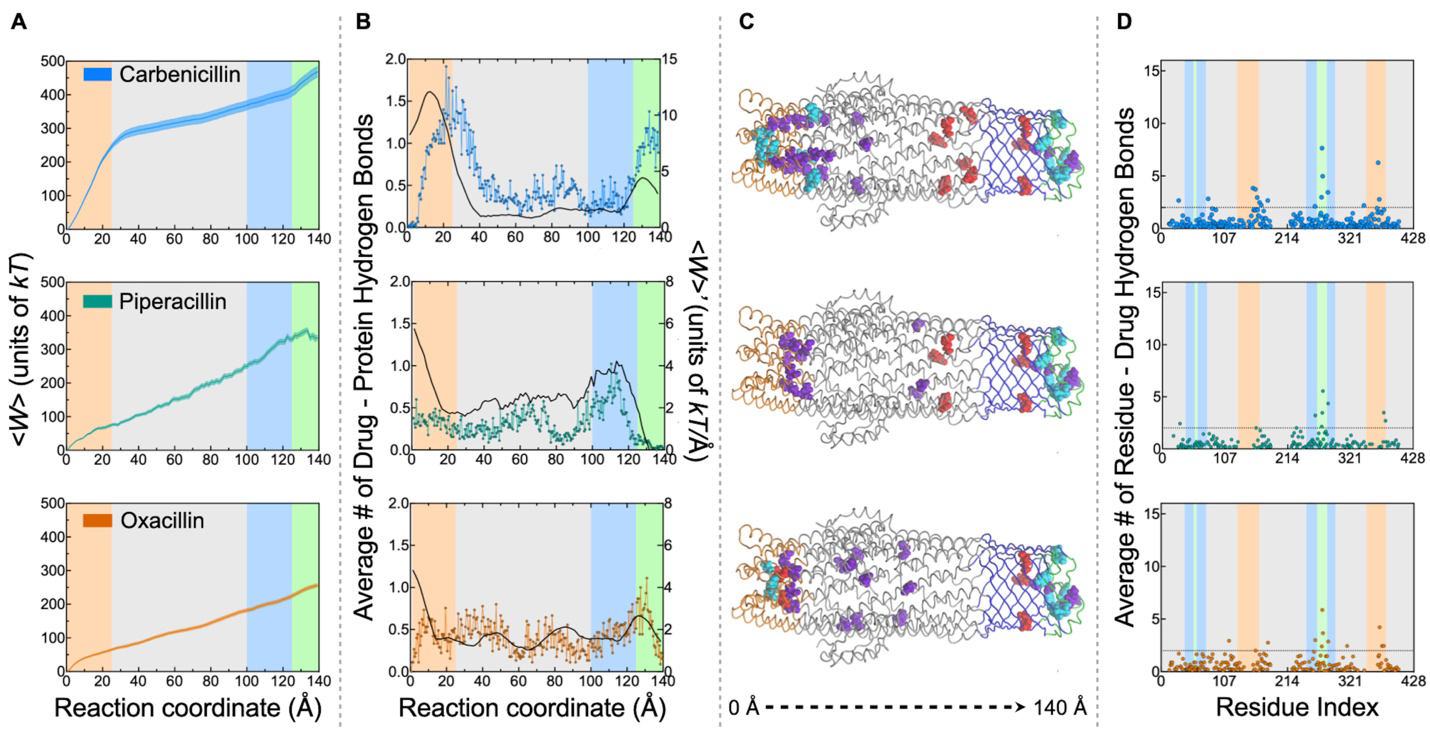
Next, we performed a feat of experimental saturation mutagenesis, mutating every single residue of TolC and testing how each mutant fared under antibiotic stress. Most mutations left TolC’s function intact. But a handful, especially near the channel’s entrance and exit, crippled efflux and made bacteria dramatically more vulnerable.
One mutation, D371G, was particularly revealing. It disrupted a set of salt bridges that normally choreograph the channel’s opening. Rather than freeing the passage, the mutation caused drugs to linger, effectively jamming the pump. We describe this as an allosteric occlusion effect, a local tweak that ripples through the protein’s long body and alters function at a distance.
Taken together, the results paint TolC as an evolutionarily optimized machine: tuned for efficiency, but vulnerable at specific hinges and gates. The work also points to a strategy for antibiotic design: target the weak spots at TolC’s periplasmic entryway, where drugs first encounter the pump. By crafting molecules that bind or stall here, scientists may be able to sidestep efflux and restore antibiotic potency.
As we may put it, understanding TolC’s shifting architecture isn’t just a matter of molecular curiosity. It could be the key to slowing the relentless arms race between bacteria and modern medicine, one salt bridge, one channel opening at a time.